Developmental and Hyperactive Ras Tumor (DHART) SPORE
With a worldwide incidence of 1 in 3000, neurofibromatosis type 1 (NF1) is the most common inherited cancer predisposition syndrome and the founding member of a group of developmental disorders that are collectively termed “Rasopathies”. NF1 is of exceptional importance in cancer biology because it encodes a GTPase activating protein (GAP) called neurofibromin that binds to active Ras-GTP and terminates signaling by accelerating guanine nucleotide hydrolysis. Clinical manifestations of NF1 include pigmented skin lesions, skeletal dysplasia, learning disabilities, and an increased risk of developing specific neoplastic diseases that progress to aggressive cancers. These premalignant and malignant tumors frequently affect children, adolescents, and young adults (AYAs). A genetic hallmark of NF1-associated tumors is somatic loss of the normal NF1 allele. Collectively, the tumors that develop in NF1 patients are a substantial cause of morbidity and premature mortality. In addition to its role as an initiating mutation in NF1-associated cancers, recent genome-wide sequencing studies uncovered frequent somatic NF1 mutations in glioblastoma (GBM), acute myeloid leukemia (AML), melanoma, lung adenocarcinoma, and other sporadic cancers. Investigating NF1-associated tumors represents a unique opportunity to interrogate therapeutic responses and mechanisms of intrinsic and acquired drug resistance in cancers that are initiated by a mutation that directly enhances Ras output.
The Developmental and Hyperactive Ras Tumor (DHART) SPORE was funded in 2015 to pursue the overall goal of implementing effective targeted molecular therapies for neoplasms and cancers characterized by germline and somatic NF1 mutations. NF1 is the most common inherited cancer predisposition syndrome. In contrast to most other SPORE efforts supported by the NCI, the program does not focus on a single cancer. Instead, we are working to implement new treatments for (“orphan”) cancers in a common biochemical pathway that develop and progress in different tissues due to NF1 mutations.
The overall goal of this Developmental and Hyperactive Ras Tumor (DHART) SPORE is to address this unmet medical need by performing integrated, mechanistically based translational research that will lead to effective targeted molecular therapies for neoplasms and cancers characterized by NF1 mutations. The objectives of this renewal application are:
-
To evaluate novel mechanism-based therapies for NF1-associated tumors in validated preclinical models and in clinical trials that emphasize on discovering biomarkers predictive of drug response;
-
To delineate the comparative molecular and phenotypic signatures of germline vs sporadic mutations of NF1.
-
3. To decrease tumor associated morbidity and mortality of patients with NF1.
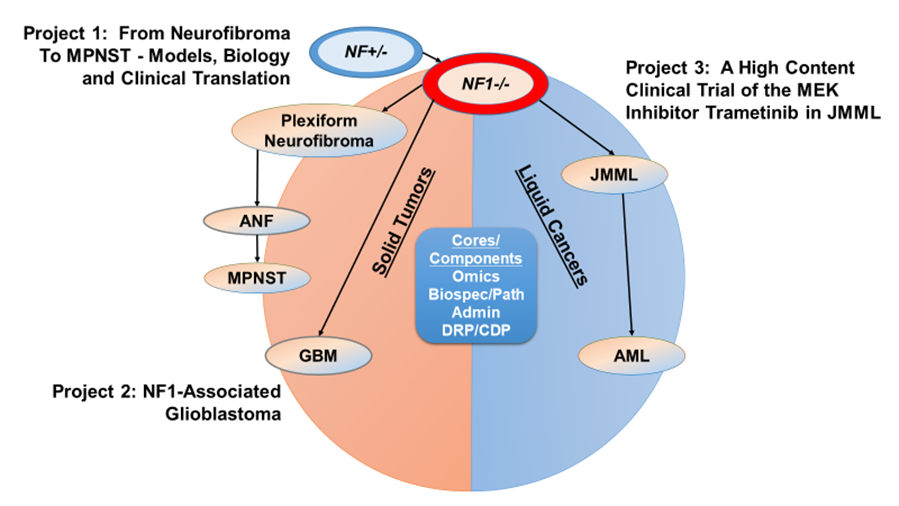
The DHART SPORE is a national collaborative effort that harnesses the expertise of researchers at the Pediatric Branch of the National Cancer Institute and six leading academic institutions. This team is addressing central issues for implementing mechanism-based treatments for rare tumors driven by hyperactive Ras signaling in the pediatric, adolescent, and young adult (AYA) population. Our program encompasses three highly integrated projects and three cores. As depicted in Figure 1, the projects are:
-
Project 1: From Neurofibroma to MPNST: Models, Biology and Translation to Clinic.
-
Project 2: NF1-associated Glioblastoma
-
Project 3: A High Content Clinical Trial of the MEK inhibitor Trametinib in JMML
The cores support the research projects, developmental research projects, and career development investigators to facilitate and expand translational research for the SPORE research efforts. The Administrative Core (Core A) provides scientific and fiscal oversight for the program and curates an integrated SPORE database in which all preclinical and clinical trial data are stored and shared. Core A leverages the expertise of internal and external advisors, superb environments for conducting translational research across multiple NCI-funded Comprehensive Cancer Centers, and strong institutional commitments.
There are two state-of-the-art research core research facilities. The Biospecimen/Pathology Core (Core C) receives human and mouse samples from all three SPORE projects, and also performs pathological review of these samples. The Omics Core (Core B) provides state-of-the-art support for research design consultation, performance of genomics and kinomics experiments, and integrated data analysis to DHART investigators to elucidate changes during NF1-related tumor development and in response to therapy.
Given the central importance of aberrant Ras/GAP function in human cancer, achieving the goals of this SPORE will broadly advance translational cancer research and treatment. The work proposed in the DHART SPORE, thus, has implications for understanding the risks and benefits of current treatment options in NF1 patients and, more broadly, for enhancing the treatment of the ~1/3rd of cancers with somatic RAS mutations.